
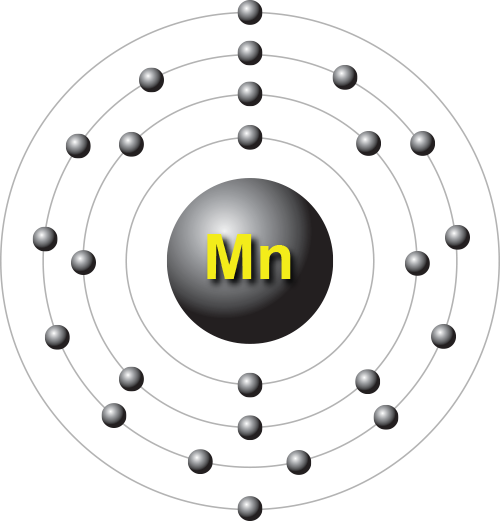
In the 17th century, German chemist Johann Glauber first produced permanganate, a useful laboratory reagent (although some people believe that it was discovered by Ignites Kaim in 1770). Some speculate that the exceptional hardness of Spartan steels derives from the inadvertent production of an iron-manganese alloy. Manganese can be found in the iron ores used by the Spartans. The Egyptians and Romans used manganese compounds in glass-making, to either remove color from glass or add colour to it. Manganese (apparently from Latin magnes, meaning "magnet") was in use in prehistoric times paints that were pigmented with manganese dioxide can be traced back 17,000 years. In minor applications, (e.g., manganese phosphating), zinc and sometimes vanadium are viable substitutes. Substitutes: Manganese has no satisfactory substitute in its major applications. No practical technologies exist for replacing manganese with other materials or for using domestic deposits or other accumulations to reduce the complete dependence of the United States on other countries for manganese ore. The overall level and nature of manganese use in the United States is expected to remain about the same in the near term. It is very occasionally used in coins the only United States coins to use manganese were the "Wartime" nickel from 1942–1945, and the Sacagawea Dollar (2000–present). Manganese phosphating is used for rust and corrosion preventation on steel. Potassium permanganate is a potent oxidizer and used in chemistry and in medicine as a disinfectant. Manganese oxide is a brown pigment that can be used to make paint and is a component of natural umber. Manganese is used to decolorize glass (removing the greenish tinge that presence of iron produces) and, in higher concentration, make violet-colored glass. Manganese dioxide is also used as a reagent in organic chemistry for the oxidation of benzylic alcohols (i.e. Manganese(IV) oxide (manganese dioxide) is used in the original type of dry cell battery. It is also added to gasoline in order to reduce engine knocking. Among a variety of other uses, manganese is a key component of low-cost stainless steel formulations and certain widely used aluminium alloys. Steelmaking, including its ironmaking component, has accounted for most manganese demand, presently in the range of 85% to 90% of the total demand. Manganese is essential to iron and steel production by virtue of its sulfur-fixing, deoxidizing, and alloying properties. The final data column compares this percentage against the percentage of all minerals that contain the element listed in each row.Manganese ( IPA: /ˈmaŋgəniːz/, /ˈmæŋgəniːz/) is a chemical element in the periodic table that has the symbol Mn and atomic number 25. The second data column lists this number as a percentage of all minerals listed with Manganese. The first data column contains the total number of minerals listed with Manganese and the element listed for that row. Note that unlike other sections on this page this includes non-essential elements. This table compares the known valid mineral species listed listed with Manganese and the other elements listed based on the official IMA formula. Mn 2+ is essential to nutrition of at least some vertebrates ('essential minerals').Ītom mole fraction relative to Si=1 (% uncertainty)Įlement association of Manganese in the Mineral World Mn 2+ solute can be a limiting nutrient in the growth of bacteria. Mn 4+ is concentrated in deep-sea ferromanganese nodules relative to seawater. Mn 3+ is concentrated in deep-sea ferromanganese nodules relative to seawater. Mn 4+ is commonly concentrated in residual soils and sediments. Mn 3+ is commonly concentrated in residual soils and sediments. Mn 2+ enters early-forming phases in igneous rocks. Mn 4+ enters early-forming phases in igneous rocks. Mn 3+ enters early-forming phases in igneous rocks. Mn 2+ was one of the ions least depleted from the mantle in the formation of the crust. The green colour in freshly-mined spodumene (eg from the Oceanview Mine) is due to the 4+ ion, but it is rapidly reduced in sunlight to 3+ causing a colour change to pink.Ħ17 valid species containing essential Manganese It also causes the purple colour in charoite. It can also give a green colour to andalusite and in a tremolite from New York it produces a violet colour. In red muscovite from brazil, red beryl from Utah, piemontite from Whitewater, California. In a tetrahedral site such as in willemite it can give a yellow-green colour.Ĭauses red and green colours in octahedral sites. Usually gives a pink to red colour to minerals, such as rhodonite and rhodochrosite. Manganese as a chromophore in minerals and gems Wikipedia WebElements Los Alamos National Laboratory Theodore Gray's


 0 kommentar(er)
0 kommentar(er)
